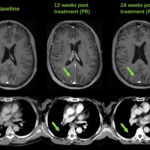
- In 67 ROS1 TKI-naïve patients, the confirmed objective response rate (cORR) and disease control rate (DCR) were 92.5% and 95.5%, respectively.
- In 38 crizotinib-pretreated patients, the cORR and DCR were 50% and 78.9%, respectively.
- In 12 patients with brain metastasis and measurable brain lesions at baseline, the intracranial cORR and intracranial DCR were 91.7% and 100%, respectively.
- In 5 patients with ROS1 G2032R mutation, 4/5 achieved confirmed partial response (cPR), and 1/5 achieved stable disease (SD).
- Taletrectinib was generally well tolerated. Low incidence of neurological adverse events (AEs) was observed, likely reflecting taletrectinib’s selective inhibition of ROS1 over tropomyosin receptor kinase B (TRKB).
NEW YORK–(BUSINESS WIRE)–#ASCO2022—AnHeart Therapeutics (“AnHeart”), a clinical-stage global biopharmaceutical company committed to developing novel precision oncology therapeutics, and Innovent Biologics, Inc. (“Innovent”) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology and other major diseases, jointly announced today updated efficacy and safety data from the Phase 2 TRUST clinical trial of taletrectinib in patients with ROS1-positive non-small cell lung cancer (NSCLC), at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting.
The Efficacy and Safety of Taletrectinib in TKI-naïve or Crizotinib-pretreated ROS1-positive Non-Small Cell Lung Cancer (NSCLC) Patients
Poster Presentation, Abstract #: 8572
The ongoing TRUST study (NCT04395677) is a multicenter, open-label, single-arm, Phase 2 study of taletrectinib in Chinese ROS1-positive NSCLC patients who are ROS1 tyrosine kinase inhibitor (TKI)-naive or crizotinib-pretreated.
As of February 23, 2022, the Phase 2 TRUST study has enrolled 67 TKI-naive and 42 crizotinib-pretreated patients. The patients were treated with taletrectinib 600 mg once daily and evaluated by independent review committee (IRC) for key efficacy endpoints including objective response rate (ORR), duration of response (DOR), disease control rate (DCR), intracranial objective response rate (IC-ORR), intracranial disease control rate (IC-DCR), progression-free survival (PFS), overall survival (OS), and safety.
- In ROS1 TKI-naïve patients, the cORR was 92.5% (62/67), including 2 confirmed complete response (cCR); and DCR was 95.5% (64/67).
- In crizotinib-pretreated patients, the cORR was 50% (19/38), DCR was 78.9% (30/38).
- Of the 5 crizotinib-pretreated patients who had ROS1 G2032R mutation, 4 achieved cPR, and 1 achieved SD.
- Of the 12 patients with brain metastasis and measurable brain lesions at baseline, the IC-ORR and IC-DCR were 91.7% and 100%, respectively. The brain tumors disappeared completely in one patient who had only non-measurable brain lesions at baseline.
- Taletrectinib was generally well tolerated. Most treatment emergent adverse events (TEAEs) were Grade 1 or 2. The most frequently reported treatment-related adverse events (TRAEs) for patients on taletrectinib were low-grade diarrhea and transient AST/ALT elevation without increase in bilirubin. Low incidence of neurological AEs was reported. The selective inhibition of ROS1 over TRKB by taletrectinib may help significantly reduce TRKB-related CNS adverse events. Some common adverse events that are frequently reported in other ROS1 inhibitors, such as vision disorders, edema, headache, dizziness, and musculoskeletal disorders were observed less frequently in taletrectinib.
“Taletrectinib is a potential best-in-class next-generation ROS1 inhibitor that is a much-needed new option to treat both ROS1-TKI-naïve and pre-treated NSCLC patients,” said Dr. Caicun Zhou, primary investigator and chief oncologist at Shanghai Pulmonary Hospital. “The TRUST study showed high objective response rates in both the first-line and second-line settings in ROS1-positive NSCLC, with excellent potency against crizotinib-resistant mutations, including G2032R solvent front mutation. We’re excited to see that taletrectinib has also demonstrated intracranial antitumor activity in patients with brain metastases.”
“Taletrectinib reported better brain penetration and intracranial activity in reference to other ROS1 inhibitors, with a favorable safety profile,” said Dr. Bing Yan, Global Chief Medical Officer and Co-Founder of AnHeart. “We look forward to advancing taletrectinib, as we believe it is a potential best-in-class next-generation ROS1 inhibitor for both ROS1 TKI-naïve and ROS1 TKI-pretreated NSCLC patients, who are in need for new therapeutic options that have antitumor activity against resistant mutations and brain metastases.”
“The updated ORR and DCR data of taletrectinib demonstrated its potential superior benefits in terms of both efficacy and safety for Chinese patients with ROS1-positive NSCLC,” said Dr. Hui Zhou, Senior Vice President of Innovent. “We are encouraged by the results and will move towards further clinical development of taletrectinib to explore the potential of the next-generation ROS1 inhibitor and benefit more NSCLC patients in the future.”
ROS1 oncogenic fusions are observed in ~1-2% NSCLC patients as well as in cholangiocarcinoma, glioblastoma, ovarian, gastric, and colorectal cancers. CNS metastasis occurs in 20-30% ROS1 TKI-naïve and in up to 50% of crizotinib-pretreated ROS1-positive NSCLC patients. Resistance to first-generation ROS1 inhibitors often occurs with secondary mutations such as ROS1 G2032R solvent front mutation, for which no FDA-approved therapy is available.
Taletrectinib is a next-generation, CNS-penetrant, selective ROS1 inhibitor. In March 2022, the NMPA grants Breakthrough Therapy Designation (BTD) to taletrectinib for both first-line TKI-naïve and second-line TKI-pretreated patients with ROS1-positive NSCLC.
A separate global Phase 2 trial TRUST-II (NCT04919811) is actively enrolling patients at clinical sites in North America, Europe and Asia. The design of the TRUST-II study is presented in the poster (#TPS8601) at ASCO 2022.
ABOUT TALETRECTINIB
Taletrectinib1 is a novel best-in-class next-generation ROS1 inhibitor designed to effectively target ROS1 fusions with potential to treat both TKI-naïve and pre-treated patients. ROS1 rearrangement is estimated to be an oncogenic driver in approximately 1 to 2 percent of patients with NSCLC. ROS1 fusions are also observed in several other cancers such as cholangiocarcinoma, glioblastoma, ovarian, gastric, and colorectal cancers. Taletrectinib has demonstrated excellent potency against crizotinib resistance, good brain penetration and intracranial antitumor activity, and favorable safety profiles in ROS1 fusion-positive NSCLC patients. In these patients, few neurological adverse events were observed, which likely benefits from the selective inhibition of ROS1 over TRKB by taletrectinib. More information about the ongoing China TRUST (Taletrectinib ROS1 LUng STudy) phase 2 trial and the global TRUST-II phase 2 trial may be found by searching clinical trial identifiers NCT04395677 and NCT04919811, respectively at https://clinicaltrials.gov. For questions about the ongoing trials, please contact [email protected].
ABOUT ANHEART THERAPEUTICS
AnHeart Therapeutics (“AnHeart”), a Cayman Islands entity (registered name AnBio Therapeutics Ltd.), is a clinical-stage global biopharmaceutical group company developing a broad pipeline of novel precision oncology therapeutics with high unmet medical needs. Its lead asset, taletrectinib, is a best-in-class next-generation ROS1 inhibitor currently in Phase 2 trials for ROS1 TKI-naïve and TKI-pretreated patients with ROS1 fusion-positive non-small cell lung cancer (NSCLC). The Company’s pipeline also includes AB-218, a mIDH1 inhibitor in Phase 2 trials with good brain penetration for multiple solid tumors with mIDH1 mutations and AB-329, an AXL inhibitor in Phase 1 studies to be used in combination with checkpoint inhibitor or chemotherapies in NSCLC or other solid tumors. The Company operates from offices in the US and China. For more information, please visit https://www.anhearttherapeutics.com/.
ABOUT INNOVENT
Inspired by the spirit of “Start with Integrity, Succeed through Action,” Innovent’s mission is to develop, manufacture and commercialize high-quality biopharmaceutical products that are affordable to ordinary people. Established in 2011, Innovent is committed to developing, manufacturing and commercializing high-quality innovative medicines for the treatment of cancer, autoimmune, metabolic, ophthalmology and other major diseases. On October 31, 2018, Innovent was listed on the Main Board of the Stock Exchange of Hong Kong Limited with the stock code: 01801.HK.
Since its inception, Innovent has developed a fully integrated multi-functional platform which includes R&D, CMC (Chemistry, Manufacturing, and Controls), clinical development and commercialization capabilities. Leveraging the platform, the company has built a robust pipeline of 32 valuable assets in the fields of cancer, autoimmune, metabolic, ophthalmology and other major therapeutic areas, with 7 products approved for marketing in China – TYVYT® (sintilimab injection), BYVASDA® (bevacizumab biosimilar injection), SULINNO® (adalimumab biosimilar injection), HALPRYZA® (rituximab biosimilar injection) , Pemazyre® (pemigatinib oral inhibitor) and olverembatinib (BCR-ABL TKI) and Cyramza® (ramucirumab), 1 asset under NMPA NDA review, 5 assets in Phase 3 or pivotal clinical trials, and an additional 19 molecules in clinical studies.
Innovent has built an international team with advanced talent in high-end biological drug development and commercialization, including many global experts. The company has also entered into strategic collaborations with Eli Lilly and Company, Adimab, Incyte, MD Anderson Cancer Center, Hanmi and other international partners. Innovent strives to work with many collaborators to help advance China’s biopharmaceutical industry, improve drug availability and enhance the quality of the patients’ lives. For more information, please visit: www.innoventbio.com. and www.linkedin.com/company/innovent-biologics/.
Note:
TYVYT® (sintilimab injection) is not an approved product in the United States.
BYVASDA® (bevacizumab biosimilar injection), SULINNO®, and HALPRYZA® (rituximab biosimilar injection) are not approved products in the United States.
TYVYT® (sintilimab injection, Innovent)
BYVASDA® (bevacizumab biosimilar injection, Innovent)
HALPRYZA® (rituximab biosimilar injection, Innovent)
SULINNO® (adalimumab biosimilar injection, Innovent)
Pemazyre® (pemigatinib oral inhibitor, Incyte Corporation). Pemazyre® was discovered by Incyte Corporation and licensed to Innovent for development and commercialization in Mainland China, Hong Kong, Macau and Taiwan.
CYRAMZA® (ramucirumab, Eli Lilly). Cyramza® was discovered by Eli Lilly and licensed to Innovent for commercialization in Mainland China.
Innovent’s Forward-Looking Statements
This news release may contain certain forward-looking statements that are, by their nature, subject to significant risks and uncertainties. The words “anticipate”, “believe”, “estimate”, “expect”, “intend” and similar expressions, as they relate to Innovent Biologics, Inc. (“Innovent” or “Company”) , are intended to identify certain of such forward-looking statements. The Company does not intend to update these forward-looking statements regularly.
These forward-looking statements are based on the existing beliefs, assumptions, expectations, estimates, projections and understandings of the management of the Company with respect to future events at the time these statements are made. These statements are not a guarantee of future developments and are subject to risks, uncertainties and other factors, some of which are beyond the Company’s control and are difficult to predict. Consequently, actual results may differ materially from information contained in the forward-looking statements as a result of future changes or developments in our business, the Company’s competitive environment and political, economic, legal and social conditions.
The Company, the Directors and the employees of the Company assume (a) no obligation to correct or update the forward-looking statements contained in this site; and (b) no liability in the event that any of the forward-looking statements does not materialise or turn out to be incorrect.
1 In June 2021, AnHeart Therapeutics and Innovent Biologics entered into an exclusive license agreement for the co-development and commercialization of taletrectinib in Greater China, including mainland China, Hong Kong, Macau and Taiwan.
Contacts
AnHeart Therapeutics
Investor Contact:
Weiqing Wang, PhD
[email protected]
212-466-6378
Media Contact:
Kimberly Ha
KKH Advisors
917-291-5744
[email protected]
Innovent Biologics
Media:
[email protected]
+86 512-6956 6088
Investors:
[email protected]
+86 512-6956 6088