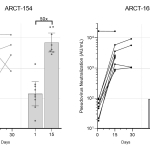
Preliminary data from ongoing clinical booster study of ARCT-154 (5 mcg) shows a 50-fold increase in neutralizing antibody geometric mean concentration against SARS-CoV-2 using a validated pseudovirus microneutralization (MNT) assay
Additional data shows activity against several variants of concern and variants of interest upon boosting with ARCT-154 (5 mcg) and ARCT-165 (5 mcg) in a surrogate virus neutralization (sVNT) assay
Company to evaluate sera from ARCT-154 and ARCT-165 vaccinated participants for activity against the omicron variant; initial data anticipated Q1 2022
SAN DIEGO–(BUSINESS WIRE)–$ARCT #ClinicalTrial–Arcturus Therapeutics Holdings Inc. (the “Company”, “Arcturus”, Nasdaq: ARCT), a leading clinical-stage messenger RNA medicines company focused on the development of infectious disease vaccines and significant opportunities within liver and respiratory rare diseases, today announced new data and updates from clinical development programs for ARCT-154 and ARCT-165, its investigational, next-generation, self-amplifying mRNA vaccine candidates targeting variants of concern.
ARCT-154 and ARCT-165 are being studied in a Phase 1/2 trial, sponsored by Arcturus, in U.S. and Singapore. The study is evaluating Arcturus’ COVID vaccine candidates as both a primary vaccination series and as a booster following initial vaccination with Comirnaty®. ARCT-154 is also currently being studied in an ongoing pivotal trial in Vietnam, sponsored and funded by Arcturus’ collaborator Vinbiotech.
Preliminary immunogenicity results from the first eight of 12 participants administered ARCT-154 and the first nine of 12 administered ARCT-165 as a booster following initial vaccination with Comirnaty® in the ongoing Phase 1/2 study demonstrates an encouraging increase in neutralizing antibody titers following booster vaccination, as measured by a validated pseudovirus (D614G variant) microneutralization (MNT) assay as well as in an exploratory surrogate virus neutralization assay (sVNT) assessing responses to multiple variants of concern and variants of interest, including the alpha, beta, gamma and delta strains.
“The booster data showing a rise in neutralizing antibody levels and potentially broad coverage across variants, while preliminary, is encouraging and provides support for the continued development of our next-generation self-amplifying mRNA vaccine candidates as differentiated, low-dose vaccines that may be effective boosters for continued prevention of infections caused by variants of concern,” said Joseph Payne, President and CEO of Arcturus Therapeutics. “We await the data from the pivotal Phase 1/2/3 study of ARCT-154 and anticipate commencing the submission of regulatory documents to the Ministry of Health in Vietnam this month, with completion of Emergency Use Authorization application in the first quarter of 2022, assuming positive data.”
In the ARCT-154 and ARCT-165 arms of the booster cohort of the ongoing Phase 1/2 study being conducted in the U.S. and Singapore, 24 participants divided into two equal groups of 12 received 5 micrograms of ARCT-154 or ARCT-165 following primary vaccination with Comirnaty® at least 5 months earlier. All participants in the booster trial were below 65 years of age at the time of receiving the booster dose. Figures 1 and 2 show the Day 15 and Day 29 post-boost results from pseudovirus microneutralization (MNT) and surrogate virus neutralization (sVNT) assays, respectively, performed with sera from the first eight participants in the ARCT-154 group, and from the first nine participants in the ARCT-165 group. The company anticipates updating the results as subsequent data become available in the coming weeks.
Arcturus and Vinbiotech anticipate commencing the submission of regulatory documents to the Vietnam Ministry of Health (MoH) this month for emergency use authorization (EUA) application for ARCT-154. Contingent upon additional requirements of the MoH, the submission is anticipated to be completed in the first quarter of 2022.
Arcturus also plans to evaluate sera from ARCT-154 and ARCT-165 vaccinated participants for activity against the SARS-CoV-2 omicron variant and expects to obtain preliminary data in the first quarter of 2022.
About Arcturus Therapeutics
Founded in 2013 and based in San Diego, California, Arcturus Therapeutics Holdings Inc. (Nasdaq: ARCT) is a clinical-stage mRNA medicines and vaccines company with enabling technologies: (i) LUNAR® lipid-mediated delivery, (ii) STARR™ mRNA Technology and (iii) mRNA drug substance along with drug product manufacturing expertise. Arcturus’ diverse pipeline of RNA therapeutic and vaccine candidates includes mRNA vaccine programs for SARS-CoV-2 (COVID-19) and influenza, and other programs to potentially treat ornithine transcarbamylase (OTC) deficiency, and cystic fibrosis along with partnered programs including glycogen storage disease type III (GSD III), hepatitis B virus (HBV), and non-alcoholic steatohepatitis (NASH). Arcturus’ versatile RNA therapeutics platforms can be applied toward multiple types of nucleic acid medicines including messenger RNA, small interfering RNA, replicon RNA, antisense RNA, microRNA, DNA, and gene editing therapeutics. Arcturus’ technologies are covered by its extensive patent portfolio (with patents and patent applications issued in the U.S., Europe, Japan, China and other countries). Arcturus’ commitment to the development of novel RNA therapeutics has led to collaborations with Janssen Pharmaceuticals, Inc., part of the Janssen Pharmaceutical Companies of Johnson & Johnson, Ultragenyx Pharmaceutical, Inc., Takeda Pharmaceutical Company Limited, CureVac AG, Duke-NUS Medical School, and the Cystic Fibrosis Foundation. For more information visit www.ArcturusRx.com. In addition, please connect with us on Twitter and LinkedIn.
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding strategy, future operations, the expectations for or likelihood of success of any collaborations, the likelihood of success (including safety and efficacy) of the Company’s pipeline (including ARCT-154 and ARCT-165), the Company’s efforts to develop a vaccine against COVID-19 and therapeutic potential thereof based on the Company’s mRNA therapeutics, the planned initiation, design or completion of experimental or preclinical work (including the evaluation of vaccine candidates against the omicron variant and other COVID-19 variants), the planned initiation, design or completion of clinical trials (including the timely completion of the Phase 1/2/3 study of ARCT-154), the likelihood that the Company will obtain clearance from regulatory authorities to proceed with future planned clinical trials, the likelihood that subsequent data from a study will be consistent with preliminary data (including with respect to the preliminary immunogenicity data of ARCT-154 and ARCT-165), the likelihood that preclinical or clinical data will be predictive of future clinical results or efficacy or safety of a candidate (including with respect to the immunogenicity results of ARCT-154 and ARCT-165 as a booster in the ongoing Phase 1/2 study), the ability to enroll, and timing for enrollment of, subjects in clinical trials, the timing and nature of any study results (including the results of the Phase 1/2/3 study in Vietnam), the likelihood that clinical data will be sufficient for regulatory approval or completed in time to submit an application for regulatory approval within a particular timeframe (including the timing for submission of an application for emergency use authorization in Vietnam), the likelihood or timing of any regulatory approval (including with respect to an EUA in Vietnam), the potential administration regimen or dosage, or ability to administer multiple doses of, any of the Company’s drug candidates, the likelihood that a patent will issue from any patent application, and the impact of general business and economic conditions. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing and you should not place undue reliance on such forward-looking statements. These statements are only current predictions or expectations, and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements, including those discussed under the heading “Risk Factors” in Arcturus’ most recent Annual Report on Form 10-K, and in subsequent filings with, or submissions to, the SEC, which are available on the SEC’s website at www.sec.gov. Except as otherwise required by law, Arcturus disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise.
Trademark Acknowledgements
The Arcturus logo and other trademarks of Arcturus appearing in this announcement, including LUNAR® and STARR™, are the property of Arcturus. All other trademarks, services marks, and trade names in this announcement are the property of their respective owners.
Contacts
IR and Media Contacts
Arcturus Therapeutics
Deepankar Roy, Ph.D.
(858) 900-2682
[email protected]
Kendall Investor Relations
Carlo Tanzi, Ph.D.
(617) 914-0008
[email protected]