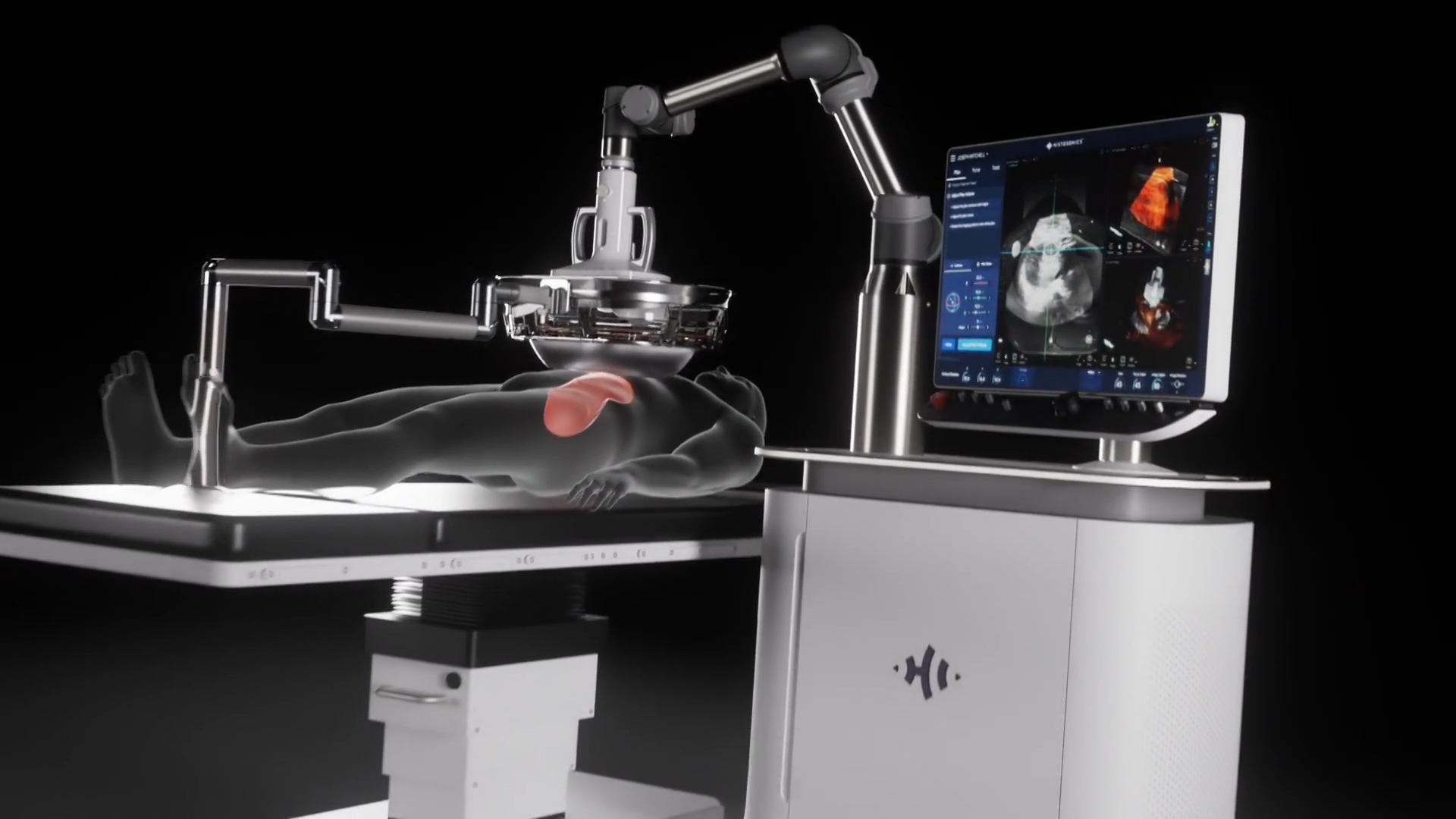
Edison Histotripsy System Selected for Competitive Accelerated Market Access Program
MINNEAPOLIS–(BUSINESS WIRE)–#HistoSonics–HistoSonics®, (www.histosonics.com), the manufacturer of the Edison® System and novel histotripsy therapy platforms, announced today that the company’s Edison System has been selected to participate in the UK’s newly created Innovative Devices Access Pathway (IDAP) Pilot Program designed to accelerate the development of cost-effective medical devices and their integration into the UK market. Applications to compete for one of the 8 available places in the highly sought after UK program began in 2023 and was open to UK and international commercial and non-commercial developers with new health technology solutions.
The IDAP Pilot Program was designed and developed by a partnership of the Department of Health and Social Care, The Medicines and Healthcare products Regulatory Agency (MHRA), The National Institute for Health and Care Excellence (NICE), NHS England, Health Technology Wales, and Scottish Health Technology Group to test the IDAP pathway and to expedite the incorporation of novel medical technologies that have demonstrated the potential to meaningful address the UK’s top healthcare concerns of today, as well as in the future. The Edison Histotripsy System was measured against four distinct eligibility criteria that were required of applicants for consideration in the IDAP program. Applicants must have met the following in order to be considered for the IDAP Pilot Program: 1) the technology addresses a life-threatening or seriously debilitating condition and there is a significant patient need, 2) the technology is innovative and transformative, 3) the technology will provide system wide benefit, and 4) the technology clearly helps to address one of the following Life Sciences Vision’s Healthcare Missions. In addition to meeting the eligibility criterion set, the IDAP program enlisted patient experts in the shortlisting and selection of the eight innovative and transformative medical devices that were eventually chosen to participate.
“Selection to the IDAP program is validation of our team’s purpose of meaningfully changing the lives of patients suffering from life-threatening conditions, starting in the liver, kidney and soon in the pancreas. The criteria set forth for the IDAP Pilot Program align extremely well with our company’s goals of providing access to innovative, non-invasive treatment to all those who may benefit from histotripsy’s unique benefits across diverse health systems,†said Mike Blue, HistoSonics CEO and President. “Our experience conducting clinical trials in the UK has provided us with both the clinical outcomes and patient experiences that fulfill the rigorous criteria set by the MHRA, and we look forward to continuing the progress we’ve made in establishing the patient benefits of histotripsy across the UK,†added Blue.
The science of histotripsy uses focused sound energy to produce controlled acoustic cavitation that mechanically destroys and liquifies targeted tissue, including tumors, at sub-cellular levels. HistoSonics’ Edison System uses proprietary technology and advanced imaging to deliver personalized, non-invasive histotripsy treatments with precision and control. The company believes that the novel mechanism of action of their proprietary technology may provide significant advantages to patients, including the ability of the treatment site to recover and resorb quickly. Uniquely, the HistoSonics’ platform also provides physicians the ability to monitor the destruction of tissue under continuous real-time visualization and control, unlike any modality that exists today.
The Edison System is indicated for the non-invasive destruction of liver tumors, including unresectable liver tumors, using a non-thermal, mechanical process of focused ultrasound.
About HistoSonics
HistoSonics is a privately held medical device company developing a non-invasive platform and proprietary sonic beam therapy utilizing the science of histotripsy, a novel mechanism of action that uses focused ultrasound to mechanically destroy and liquify unwanted tissue and tumors. The company is currently focused on commercializing their Edison System in the US and select global markets for liver treatment while expanding histotripsy applications into other organs like kidney, pancreas, and others. HistoSonics has offices in Ann Arbor, Michigan and Minneapolis, MN.
For more information please visit: www.histosonics.com/
Contacts
Josh King
Vice President of Marketing
Email: [email protected] Phone: 608.332.8124