SEOUL, South Korea–(BUSINESS WIRE)–Lemonex Inc., a biotechnology company developing innovative messenger RNA (mRNA) and siRNA therapeutics based on a novel nanoparticle drug delivery system announced on the 9th of August that an Investigational New Drug (IND) application has been approved by the Ministry of Food and Drug Safety (MFDS) for mRNA vaccine LEM-mR203 with its nano-drug delivery technology, DegradaBALL® on 21st of July.
LEM-mR203 is the first mRNA vaccine candidate to which Lemonex’s proprietary drug delivery platform technology, DegradaBALL (DegradaBALL-mRNA system), is applied. This clinical trial is for Covid-19, and safety and immunogenicity will be evaluated in healthy adults at Seoul National University Hospital, Korea.
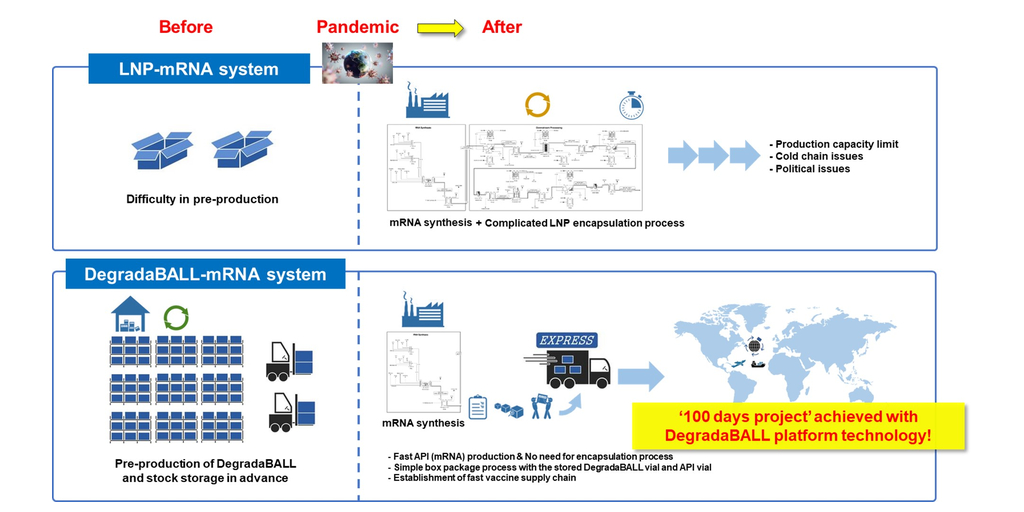
In terms of timely and effective pandemic response, mRNA vaccines have an advantage over antigen protein vaccines because they can be developed more rapidly. However, effective delivery of mRNA in the body requires drug delivery technology. The LNP that is currently applied to existing mRNA vaccines has shown limitations, such as safety concerns including anaphylaxis, myocarditis, and pericarditis, as well as the need for an ultra-low-temperature cold chain. Therefore, there is a need for next-generation drug delivery technology. The drug delivery technology of DegradaBALL developed by Lemonex may address these issues, suggesting a more advanced DDS technology for mRNA vaccines and therapeutics.
An official from Lemonex said, “LEM-mR203 is an advanced mRNA vaccine candidate harnessing DegradaBALL technology that can complement the limitations of LNP.†“We confirmed the safety and tolerability of DegradaBALL in healthy adults by successfully finishing the phase 1 clinical trial of LEM-S401 (siRNA therapy with DegradaBALL, DegradaBALL-siRNA system). IND approval of LEM-mR203 phase I clinical trial once again proved the value of the DegradaBALL technology for mRNA vaccine that improved and supplemented the limitations of LNP.â€
Another official from the company said, “mRNA vaccines and therapies will be playing an increasingly important role. However, current mRNA vaccines employing LNP pose challenges when it comes to storage, packaging, and transportation due to their formulation instability. In addition, the complicated mRNA encapsulation process should be performed immediately after mRNA synthesis in a dedicated facility, which requires further improvement for better encapsulation efficiency.†“However, unlike LNP, DegradaBALL can be pre-produced even before mRNA synthesis and stored in stock at room temperature. DegradaBALL can stabilize mRNA formulation, extend the shelf-life of the final formulation, reduce the risk of mRNA damage, and eliminate the necessity of ultra-cold chain for mRNA vaccine supply.
Currently, Lemonex is developing various competitive new drug candidates based on the DegradaBALL drug delivery platform. siRNA gene therapy LEM-S401: Successfully completed the phase 1 clinical trial in healthy human, Dual-acting RNA immuno-oncology drug LEM-S403: Production of clinical samples for clinical entry after completion of the GLP-tox studies, cytokine IL-2 therapy BALLkine-2: Completed preclinical PoC. In addition, Lemonex is strengthening its global network by signing MOUs with the International Vaccine Institute (IVI) and the mRNA vaccine technology transfer hub, the initiative supported by WHO.
In addition, Lemonex presented its DegradaBALL platform technology at the 2018 mRNA Health Conference and received an invitation to the Global Vaccine Immune Research Forum (GVIRF) hosted by the WHO, NIH, and the Bill Melinda Gates Foundation this year. Currently, the company is preparing for an IPO to list on the KOSDAQ market and seeking pre-IPO fundraising.
Contacts
Lemonex’s contact info
1. Corporate: Cheolhee Won. Ph.D.,
CEO, Lemonex, [email protected]
2. Media & Technology: Dal-Hee Min, Ph.D.,
CTO, Lemonex, [email protected]