Clinical data supports continued development of telacebec toward the first universal regimen to overcome tuberculosis regardless of drug resistance status
SEONGNAM-SI, Korea–(BUSINESS WIRE)–#Antibiotic—Qurient Co. Ltd. today announced the publication in the New England Journal of Medicine (NEJM) of positive results from the Phase 2 clinical trial for telacebec (Q203), a first-in-class, orally available antibiotic for the treatment of tuberculosis (TB).
TB is a serious bacterial infection that mainly affects the lungs and remains one of the top 10 causes of death worldwide. In 2018, approximately 10 million people were sickened by TB, and 1.5 million died. The World Health Organization reported that 484,000 people with TB showed resistance to rifampicin in 2018.
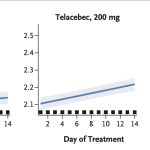
The Phase 2 clinical study for telacebec (Q203) was a prospective, randomized, open-label trial involving 61 patients with newly diagnosed, rifampicin- and isoniazid-susceptible pulmonary tuberculosis. Patients were assigned to receive 14 days of oral telacebec at a dose of 100 mg, 200 mg, or 300 mg once daily or combination therapy with rifampicin, isoniazid, pyrazinamide, and ethambutol (RHZE). Serial (16-hour) sputum samples were collected daily, and time to positivity in liquid culture was measured in hours.
Increasing doses of telacebec were associated with greater reductions in viable bacterial load in sputum. The primary endpoint data showed a daily increase in the time samples required to become culture positive after sputum inoculation. This shows that daily treatment with telacebec is able to reduce the number of live M. tuberculosis in a dose dependent manner, with greater activity for increasing doses including 100 mg, 200 mg and 300 mg daily.
Telacebec was associated with acceptable adverse-event rates, and adverse events were equally distributed among all groups. There were no serious adverse drug reactions and no adverse drug reactions that resulted in early withdrawal from the study.
This trial took place in South Africa under a U.S. IND (ClinicalTrials.gov: NCT03563599) and was led by global key opinion leaders in TB located in Cape Town, South Africa: Professor Andreas Diacon, a national principal investigator at TASK Applied Science, Doctor Veronique de Jager, principal investigator at TASK Applied Science, and Professor Rodney Dawson, principal investigator at University of Cape Town Lung Institute.
“We are very excited to achieve clinical proof-of-concept for telacebec, a new class of anti-tuberculosis agent,” said Kiyean Nam, Ph.D., CEO and CSO of Qurient.“This publication is recognized by global academics for the excellent clinical results of telacebec and provides hope to realize the first ‘100% effective regimen,’ making the distinction between drug-susceptible and drug-resistant TB obsolete.”
About Telacebec (Q203)
Telacebec is a selective inhibitor with high specificity for the cytochrome bc1 complex of Mycobacterium tuberculosis. This complex is a critical component of the electron transport chain, and inhibition disrupts the bacterium’s ability to generate energy. Telacebec has received Orphan Drug Designation and Fast Track Designation from the U.S. FDA. Telacebec also has been found effective against buruli ulcer (Mycobacterium ulcerans), a chronic, necrotizing disease that affects skin and sometimes bone and can lead to permanent deformity and long-term disability (Nature Communications, 2018).
About Qurient
Qurient is a clinical-stage biopharmaceutical company listed in Korea Exchange (KRX 115180). Qurient has two programs in clinical development and one program in the IND enabling stage. Q301 is a topical leukotriene inhibitor for atopic dermatitis in a Phase 2b clinical trial in the United States. Q702 is a Axl/Mer/CSF1R triple inhibitor for immune-oncology and drug resistant non-small cell lung cancer, Q901 is a selective CDK7 inhibitor for oncology, these two program were licensed from Max Planck Innovation and Lead Discovery Center in Germany. Telacebec (Q203), which just completed a Phase 2 clinical trial, was licensed from Institute Pasteur Korea. For more info, please visit www.qurient.com.
Contacts
Jessica Yingling, Ph.D.
Little Dog Communications Inc.
[email protected]
+1.858.344.8091